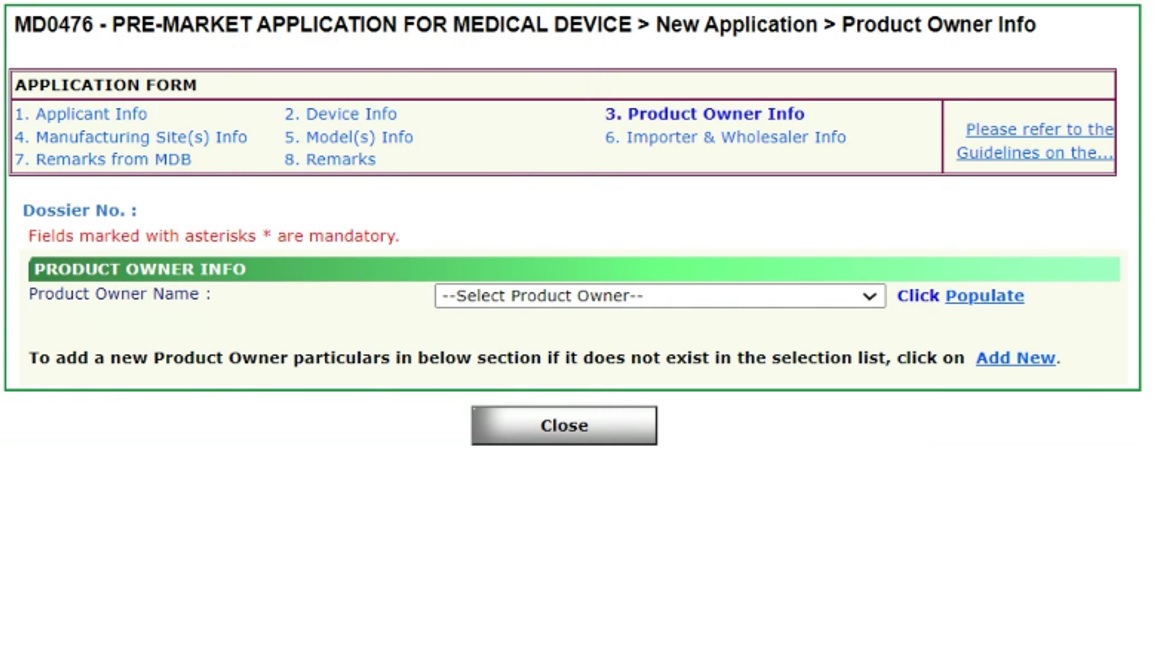
Product Owner Information in MEDICS
This is with regards to the Product Owner (PO) section in the submission of a new MEDICS Pre-market application. It has come to our attention that applicants have been creating new POs even though the same PO already exists in the PO drop down selection.
We would like to highlight that each new PO entered by applicant will result in a new PO ID being created. This is a potential issue for applicants who wish to submit a new Free Sales Certificate (FSC) and/or Change of Registrant application. Applicants would then be unable to select all SMDR device listings with the same PO name due to different PO IDs being created.
In order to amend the PO ID number to align with the other SMDR listing(s), a Change Notification (CN) submission is required to be submitted by the Registrant. The CN type to submit would be “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)” and there will be no fees chargeable. No other changes will be allowed in this category of CN application submission. Here are the instructions on how to submit this CN:
- Select “Other Change(s) - Applicable only upon receipt of email from HSA, authorising submission under this category” in MEDICS
- Under “Other Change(s)”, select “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)
- Upload the Annex 2 Summary Table of Changes in MEDICS
- Update Product Owner Code under Product Owner Info section in MEDICS for the required SMDR device listing(s)
- Submit a screenshot of the selected Product owner with the Product Owner code for verification
Going forward, we would also like to encourage applicants to first search out for existing POs prior to creating a new PO at the point of a new pre-market application submission.
Industry member, Medical devices
Published:
Subscribeto stay up to date with HSA news and regulatory updates.
Regulatory Updates