Supporting innovation and timely access to advanced therapeutics
The HSA Innovation Office is here to support the efficient development of innovative therapeutic products in Singapore. Researchers, academics, biotech, and pharmaceutical companies can seek scientific and regulatory advice for their innovative products — particularly during the early stages of development — to facilitate translation of scientific breakthroughs into impactful clinical treatments, ultimately delivering significant benefits to patients in Singapore and worldwide.
Scope of advice
We provide scientific and regulatory advice on novel investigational products that are either Therapeutic Products (TPs) or Class 2 Cell, Tissue and Gene Therapy Products (CTGTPs), at any stage of development. You may seek advice on one or more of the following:
- Non-clinical studies required to support clinical development
- Clinical trial design and clinical development plans
- Chemistry, manufacturing and controls (CMC)
- Manufacturing requirements
Request regulatory advice or meeting with us
To request regulatory advice or a meeting, please complete this form and send it with your Briefing Document to HSA_InnovationOffice@hsa.gov.sg.
Your questions should be clear and specific so that we can provide advice targeted to your questions. If your questions are too broad or general, our response may also be general. Questions should ideally be posed in a such a way that we can either agree or disagree with a proposed plan. Please refer to the HSA Guidance on Submission of IO Requests, Section 3, for examples of clear and focused questions.
Your Briefing Document should include adequate background information and product description. In addition, supporting information relevant for addressing your questions should be included. Please refer to the HSA Guidance on Submission of IO Requests, Section 4, for examples of supporting information that may be included in the Briefing Document.
Your Briefing Document should be structured, well-organised, and paginated with relevant information presented in a manner for easy reference, and ideally accompanied by a table of contents (see Suggested Format for the Briefing Document).
Please refrain from submitting separate publications as the Briefing Document; instead, summarise in the Briefing Document the pertinent and specific aspects from the publications that are relevant to the context of the questions asked. Wherever possible, summarise and interpret raw data rather than including it in its entirety. This approach will facilitate a more efficient and thorough review by HSA.
Our response timeline
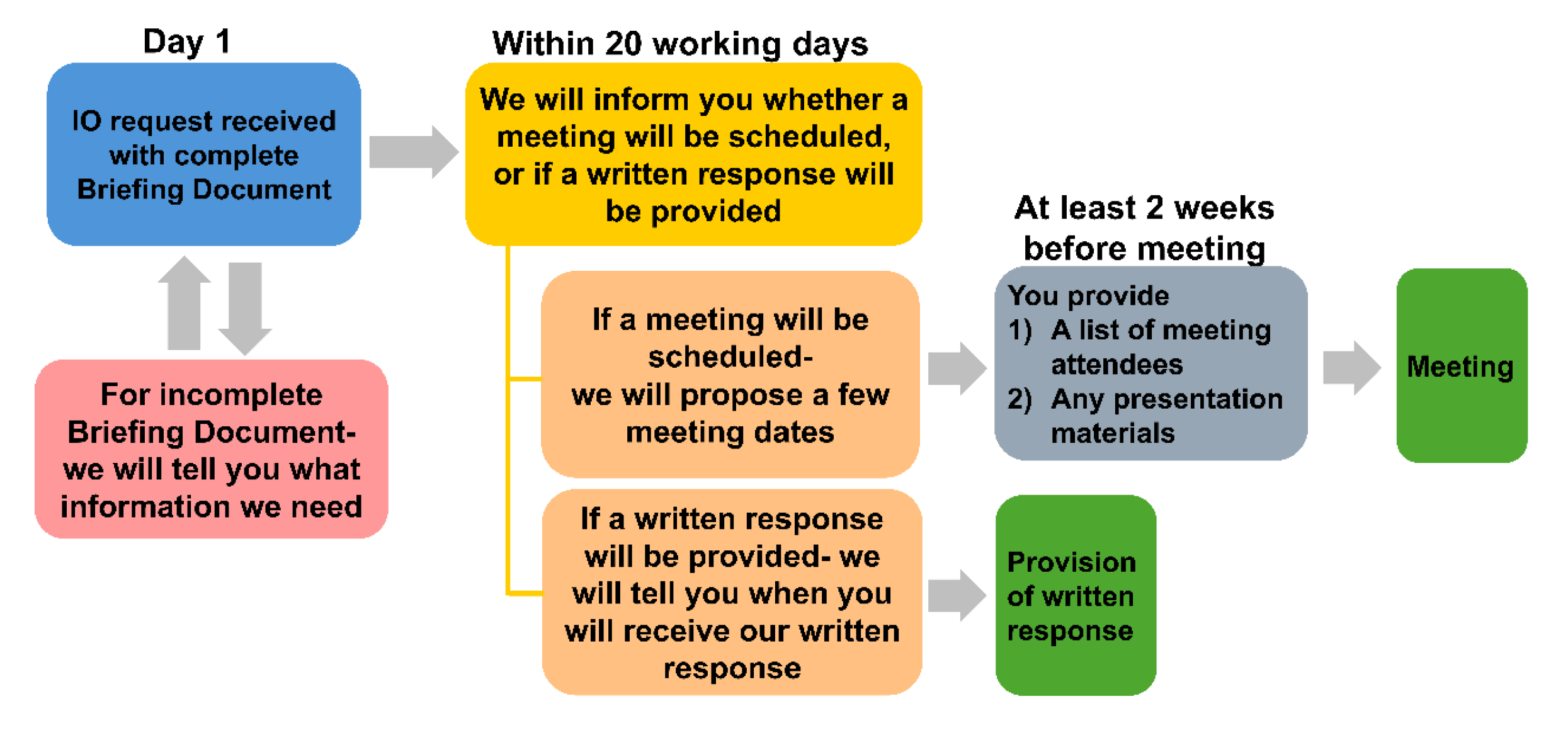
Once you have submitted your request, we will inform you within 20 working days of receiving a complete Briefing Document whether a meeting will be scheduled, or if a written response will be provided. This decision will be based on the complexity of your questions. If your request may benefit from further discussion, we may propose a meeting to discuss the details of your request in depth. The 20-day timeframe is for us to inform you whether a meeting will be arranged, or if not needed, the estimated timeline for receiving HSA’s written response. A meeting will not be scheduled during this period.
Fees
There are no fees for engagements with the Innovation Office.