Pre-submission Meeting Request
1. Purpose of Pre-submission Meeting
An applicant may request for a pre-submission meeting to seek HSA’s advice on specific issues relating to the data package for supporting an application submission, if the issues could not be addressed by the self-help mechanisms provided on this website.
A pre-submission meeting is reserved for scientific discussion and does not provide for screening or checking of the submission dossier for the applicant. To ensure the correctness of the application type and the completeness of the dossier, please refer to Guidance on Therapeutic Product Registration in Singapore and the application checklist.
2. Pre-submission Meeting Criteria
Before making a request for pre-submission meeting, the applicant must ensure that at least one of the criteria below is met:
i. The product is a novel therapeutic product developed using new or emerging technologies; or
ii. The product is developed in the absence of, or deviates from local or international regulatory guidance.
The following required documents must be submited together with the request for the pre-submission meeting:
- Proposed agenda for the meeting;
- Summary information which may include Chemistry, Manufacturing and Controls (CMC)/ non-clinical/ clinical information of the product and proposed application;
- Specific scientific issues that require advice
HSA may reject the pre-submission meeting request if:
- The product does not fulfil the pre-submission meeting criteria above; or
- The request is not accompanied by the required documents; or
- The issues can be addressed via email instead of having a pre-submission meeting
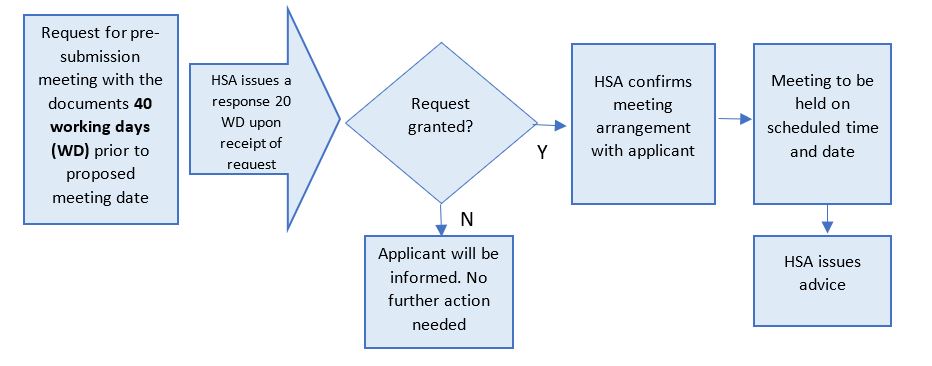
3. Overview of the Process
Points to note:
- For meetings which are held face-to-face, the venue of the meeting will be held at HSA.
- For virtual meetings, recording is not allowed.
4. Timeline
The pre-submission meeting request should be made at least 40 working days prior to the proposed meeting date through the Pre-submission Meeting Request Form.
HSA will review the request and issue a response within 20 working days form the date of the request submission.
5. How to apply
Submit your request for a pre-submission meeting using the Pre-submission Meeting Request Form.
Note: HSA will not accept any request for pre-submission meetings if the pre-submission meeting criteria specified in paragraph 2 are not fulfilled.
Changes cannot be made to the application form once submitted.