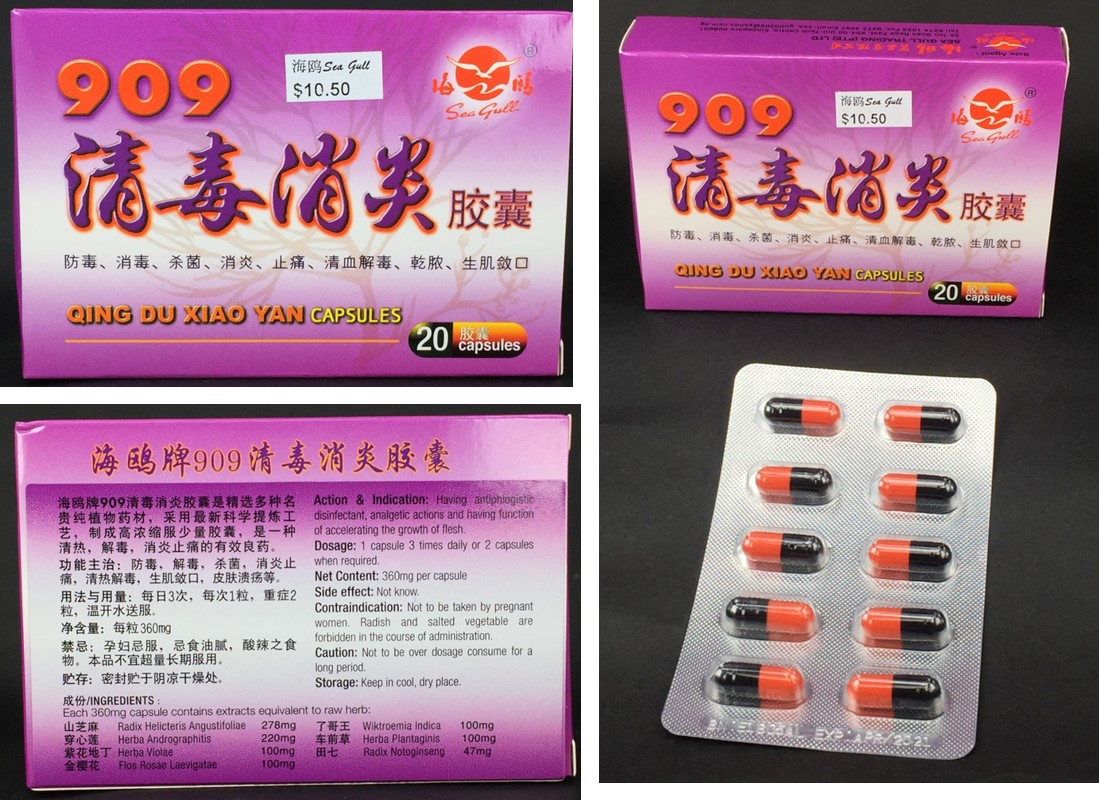
909 Qing Du Xiao Yan Capsule
| Recall No : |
PR180030 |
| Date : |
30 Nov 2018 |
| Name of Product : |
909 Qing Du Xiao Yan Capsule (909清毒消炎胶囊) |
| Batch No. : |
E1805AL |
| Expiry Date : |
04/2021 |
| Pack Size : |
300 capsules/bottle; 20 capsules/box |
| Class of Recall: |
2 |
| Level of Recall : |
Retail |
| Product Reference No: |
114030 |
| Local Company : |
Sea Gull Trading (Pte) Ltd |
| Manufacturer: |
Union Chemical and Pharmaceutical Pte Ltd |
| Country of Manufacture : |
Singapore |
| Reason(s) for Recall : |
The product was tested to contain Total Yeast and Mould Count exceeding legislative limits |
Consumer, Healthcare professional, Industry member, Chinese Proprietary Medicines
Published:
Product Recalls