Reminder on risk of extrapyramidal side effects with metoclopramide in paediatric patients aged 18 years and below
Metoclopramide has been registered in Singapore since 1989 for the prevention and treatment of nausea and vomiting due to various conditions. It inhibits dopamine receptors both centrally in the chemoreceptor trigger zone (CTZ) and peripherally in the upper gastrointestinal tract, blocks the action of serotonin at the 5-hydroxytryptamine (5-HT3) receptors in the CTZ and has prokinetic activity.1 Six registered products are available locally in various dosage forms (i.e., tablets, syrups and injections).
Since 2023, HSA has observed more cases of metoclopramide- induced extrapyramidal side effects (EPSE) in paediatric patients aged 18 years and below. Most cases (82.6%) were prescribed oral metoclopramide in the primary care setting (i.e., General Practitioners (GPs), polyclinics) for off-label uses such as vomiting secondary to gastritis, gastroenteritis or other viral illnesses. Standard adult dosing (10 mg three times daily) was commonly prescribed regardless of patient weight.
Use of metoclopramide in paediatric patients
HSA would like to remind healthcare professionals about the approved indications of metoclopramide-containing products to reduce the risk of neurological and other dose-related adverse reactions.2 Metoclopramide is contraindicated in infants less than one year old. In patients aged one to 18 years, metoclopramide is approved as a second-line treatment of established post-operative nausea and vomiting, administered via the intravenous route. The approved dose for paediatric patients is 0.10 to 0.15 mg/kg body weight, up to three times daily. The recommended maximum dose in 24 hours is 0.5 mg/kg body weight, up to 30 mg daily.
As potentially serious neurological adverse events are dose-related, healthcare professionals are recommended to use the minimum effective dose of metoclopramide. Treatment should be kept as short as possible and treatment beyond 12 weeks should be avoided unless the therapeutic benefit outweighs the risk.
Local Situation
As at 1 August 2025, HSA has identified 84 cases of metoclopramide-induced EPSE (e.g., oculogyric crisis, dystonia, akathisia and tardive dyskinesia) in paediatric patients over the past five years, with increasing number of cases observed since 2023 (Figure 1). These included reports to HSA by healthcare professionals and cases detected from electronic medical records of patients who visited the emergency department or were admitted in public hospitals. There were more reports in females (n=52, 61.9%), and the affected patients ranged from 8–18 years old (median: 16 years). Majority of the patients were Chinese (n=57, 67.8%), followed by Indians (n=14, 16.7%), Malays (n=8, 9.5%) and patients of other ethnicities (n=5, 6.0%). None were reported to have irreversible tardive dyskinesia.
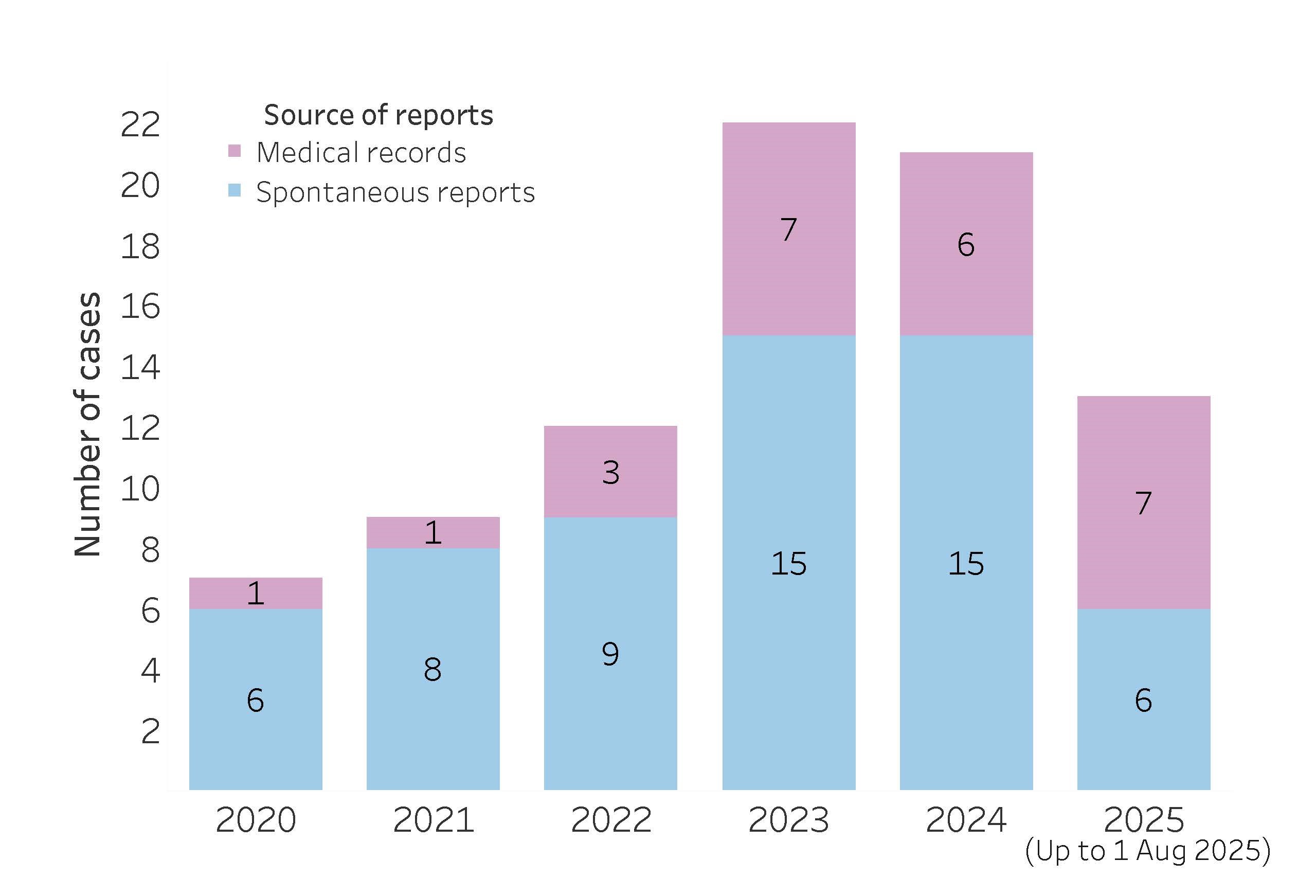
Figure 1. Local cases of metoclopramide-induced extrapyramidal side effects among paediatric patients aged 18 years and below in the past five years (January 2020 – August 2025)
Of the 69 cases with documented sources of metoclopramide, majority (n=57, 82.6%) were prescribed oral metoclopramide in the primary care setting (GPs 79.7%, polyclinics 2.9%). Seven (10.1%) patients received metoclopramide while admitted in public hospitals, with four administered intravenously and three orally. The remaining five patients self-medicated with oral metoclopramide. Majority of the patients (n=58/64, 90.6%) were prescribed metoclopramide for off-label indications such as vomiting secondary to gastritis, gastroenteritis or other viral illnesses. Patients were most frequently prescribed metoclopramide at a dose of 10 mg three times daily regardless of their weight, which ranged from 34 kg to 82 kg where documented. Notably, concurrent use of other drugs (e.g., fluoxetine, olanzapine, domperidone and prochlorperazine) in seven cases could have increased the risk of EPSE.
Choice of anti-emetics in paediatric patients
Where the use of anti-emetics is warranted, healthcare professionals could consider alternative anti-emetics for paediatric patients aged 18 years and below due to the risk of potentially serious neurological and cardiovascular adverse events with metoclopramide. The incidence of EPSE was found to be 9% (95% confidence interval 5–17%) in a meta-analysis of children administered metoclopramide although the dose of metoclopramide used in these studies varied widely.3 Risk factors for metoclopramide-induced EPSE include4:
- Use in paediatric patients
- Females
- Doses exceeding recommended doses
- Extended duration of therapy (>12 weeks)
- Kidney impairment
- Concurrent use of drugs that can cause EPSE (e.g., anti- psychotics
- Concurrent use of strong CYP2D6 inhibitors (e.g., fluoxetine, paroxetine, bupropion)
Domperidone, promethazine and ondansetron are among the anti-emetics recommended by PaedsENGAGE, a pilot programme led by KK Women’s and Children’s Hospital and National University Hospital to partner GPs across Singapore in determining the appropriate care setting for mild and moderate paediatric conditions. Recommended paediatric doses from the PaedsENGAGE drug reference guide are shown in Table 1.
Table 1. Recommended anti-emetics in the PaedsENGAGE Drug Reference Guide (1st edition)
| Drug (Route) | Paediatric dose | Usual adult dose | Remarks |
|---|
| Domperidone (Oral) | 0.25 mg/kg three times daily | 10 mg three times daily | Maximum one week duration |
| Promethazine (Oral) | 0.25–0.5 mg/kg
every 6 – 8 hours | 12.5–25 mg every four to six hours | Contraindicated in children
< two years old |
| Ondansetron (Oral / Sublingual) | 0.1–0.2 mg/kg
every 8 hours | 4–8 mg every eight to 12 hours | For use in children > six months old. Only to be given in clinic setting |
Some considerations with the use of anti-emetics in paediatric patients
Domperidone has a similar mechanism of action to metoclopramide, but it has a lower risk of EPSE as it penetrates poorly into the central nervous system.1 Promethazine, an antihistamine and dopamine antagonist, is contraindicated for use in children less than two years old due to the risk of sedation and respiratory depression. Studies have found ondansetron to be effective in cessation of vomiting, reducing the need for intravenous fluids and risk of hospitalisation, even when given as a single dose.5-7 In a meta-analysis, ondansetron was found to be more effective than domperidone in the cessation of vomiting in children with gastroenteritis.8 The use of ondansetron is generally well-tolerated, although there is mixed evidence on whether ondansetron increases the frequency of diarrhoea when used in children with gastroenteritis. It is recommended for use in the clinic setting to allow for close monitoring of the child’s hydration status and to avoid masking surgical conditions which present with persistent vomiting. In addition, multiple doses of ondansetron have been associated with the risk of QT interval prolongation, which can lead to potentially fatal cardiac arrythmias, although this has been mainly observed in adults.9 This risk is increased when ondansetron is used in high doses and in patients with risk factors such as pre-existing cardiac disease or disorders associated with electrolyte abnormalities.6
HSA’s advisory
Although EPSE is generally reversible with drug discontinuation and treatment, its unexpected occurrence can be distressing for both patients and caregivers. Healthcare professionals are encouraged to consider alternatives to metoclopramide when use of anti-emetics is required in paediatric patients, and to adopt weight-based dosing of metoclopramide for patients up to 18 years of age. Treatment duration should be limited, and both patients and caregivers should be counselled on the risk of EPSE.
References
- J Pediatr Gastroenterol Nutr 2019; 68(4): 466-71
- https://www.hsa.gov.sg/announcements/safety-alert/restrictions-on-the-use-of-metoclopramide-containing-products
- Drug Saf 2016; 39: 675-87
- UptoDate Metoclopramide; accessed 11 August 2025
- Pediatrics 2020;145(4): e20193260
- Aliment Pharmacol Ther 2016; 44(5): 438-46
- Eur J Pediatr 2020; 179(7):1007-16
- Cureus 2022;14(8): e27636
- Egypt Heart J 2023; 75(1): 56
Healthcare professional, Industry member, Therapeutic Products
Published:
Safety Alerts